A Roadmap to Successful Manufacturing Execution System Deployment
Manufacturing Execution Systems (MES) transform production processes by bridging the gap between planning systems and shop floor operations. For FDA-regulated manufacturers, proper MES implementation steps are crucial for compliance, efficiency, and operational excellence. This article outlines the essential phases and considerations for a successful MES implementation project.
Key topics covered:
- Strategic assessment and planning for MES implementation
- Critical preparation steps before deployment
- Core implementation phases and their requirements
- Post-implementation validation and continuous improvement
- Common challenges and their solutions

The Strategic Value of MES in Regulated Manufacturing
A Manufacturing Execution System serves as the operational backbone for production facilities, especially in FDA-regulated industries. Before examining the specific MES implementation steps, it’s important to understand the strategic benefits this technology delivers:
| MES Benefits | Impact on Manufacturing Operations |
| Real-time visibility | Complete transparency into production status and product genealogy |
| Compliance documentation | Automated record-keeping for regulatory requirements |
| Process standardization | Consistent execution of procedures across operations |
| Data integrity | Secure, accurate, and reliable production information |
| Resource optimization | Improved labor and equipment utilization |
For pharmaceutical and biologics manufacturers, these benefits directly impact manufacturing efficiency and regulatory compliance. A well-executed MES deployment becomes a competitive advantage in highly regulated markets.
Phase 1: Assessment and Planning
The initial MES implementation steps focus on strategic alignment and project preparation, establishing the foundation for all subsequent activities. This critical phase determines whether your MES deployment will deliver transformative value or become another costly technology project that fails to meet expectations. During assessment and planning, organizations must clearly define their current state challenges, future state vision, and the specific business outcomes the MES should enable.
For FDA-regulated manufacturers, these planning MES implementation steps must specifically address regulatory requirements including electronic record compliance, data integrity, and validation expectations. The planning phase should produce a comprehensive project charter that aligns technical requirements with business objectives and compliance needs. Organizations that invest sufficient time in this phase typically experience fewer delays and scope changes during later implementation stages.
Strategic Assessment
The foundation of successful MES implementation begins with a comprehensive assessment of current manufacturing processes. This evaluation should:
- Document existing workflows and identify pain points
- Map information flows across departments
- Analyze regulatory compliance requirements
- Establish measurable objectives for the implementation
This assessment phase guides all subsequent MES implementation steps by establishing clear benchmarks and targets.
Team Formation
An effective MES implementation requires cross-functional expertise. The core team should include:
- IT specialists with systems integration experience
- Production managers with deep process knowledge
- Quality assurance professionals familiar with pharma compliance requirements
- Executive sponsors who can drive organizational change
Many organizations partner with specialized consulting firms like GMP Pros to supplement internal capabilities during implementation.
System Selection
Choosing the appropriate MES solution ranks among the most critical MES implementation steps. Selection criteria should include:
- Alignment with industry-specific regulatory requirements
- Integration capabilities with existing systems (ERP, LIMS, etc.)
- Scalability to accommodate future growth
- Vendor experience in FDA-regulated environments
- Total cost of ownership considerations
For many pharmaceutical manufacturers, platforms that support electronic batch record implementation provide significant compliance advantages.
Phase 2: Design and Configuration
After establishing the foundation, the MES implementation steps progress to the critical design and configuration phase. This stage transforms abstract requirements into concrete system specifications that will guide the actual build-out of the solution. The design phase requires meticulous attention to detail as decisions made here will fundamentally shape how the MES functions in your FDA-regulated environment.
Effective design and configuration demand a deep understanding of both manufacturing processes and regulatory requirements. The project team must carefully balance operational efficiency with compliance needs, ensuring the resulting system supports both objectives. Many organizations find this phase particularly challenging without specialized expertise in MES implementation steps for regulated environments.
Process Mapping
Detailed documentation of current and future-state processes serves as the blueprint for configuration. This step includes:
- Identifying all production workflows that will interact with the MES
- Defining critical control points and data collection requirements
- Mapping interfaces with other manufacturing systems
- Establishing standard operating procedures (SOPs) for each process
Process mapping ensures the configured system reflects actual operational requirements rather than generic templates.
System Configuration
The configuration phase translates process requirements into system settings:
- Master data setup (materials, equipment, resources)
- Workflow definitions and business rules
- Electronic forms and data collection points
- Reporting templates and dashboards
- User roles and access controls
For FDA-regulated manufacturers, proper configuration directly impacts data quality monitoring capabilities and compliance readiness.
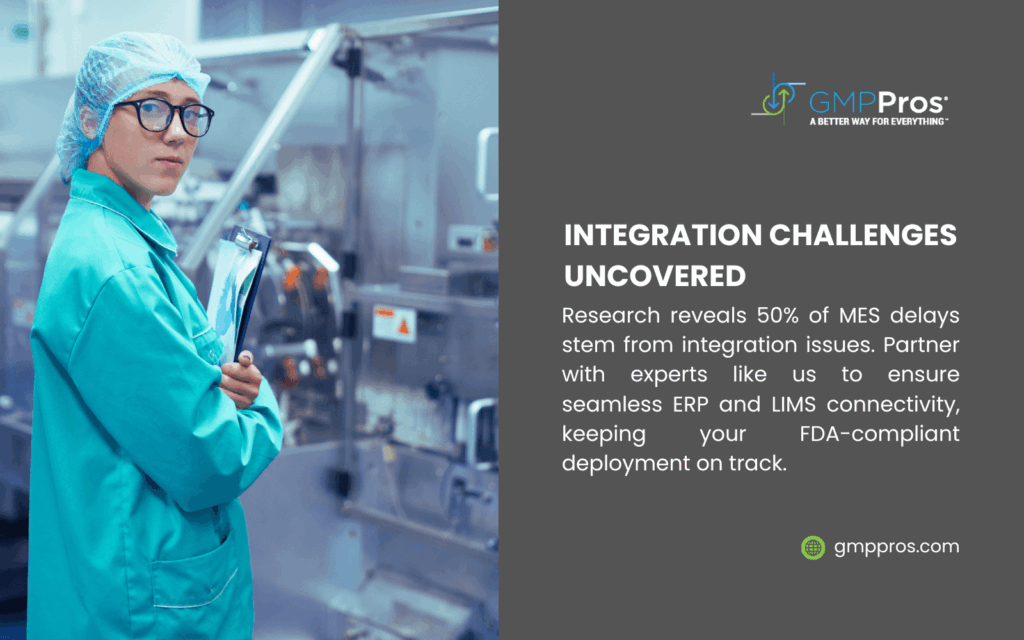
Integration Framework
Most MES implementations require integration with multiple enterprise systems:
| System Type | Integration Purpose |
| ERP | Production orders, material information, resource availability |
| LIMS | Quality specifications, test results, material release |
| Equipment | Automated data collection, machine status monitoring |
| Document Management | Procedures, specifications, training records |
A robust integration strategy ensures data flows seamlessly across the manufacturing technology ecosystem.

Phase 3: Validation and Testing
For FDA-regulated manufacturers, validation represents one of the most crucial MES implementation steps in the entire project lifecycle. This phase ensures that the configured system not only functions as intended but also meets all regulatory requirements for data integrity, security, and traceability.
The FDA expects documented evidence that systems producing electronic records are reliable and consistently perform according to predetermined specifications.
The validation phase follows a risk-based approach where critical system functions receive proportionally more rigorous testing. Each validation activity must align with the company’s overall validation master plan and comply with 21 CFR Part 11 requirements for electronic records and signatures.
The documentation created during these MES implementation steps will likely be scrutinized during regulatory inspections, making thoroughness and accuracy paramount.
Test Script Development
Comprehensive test scripts verify that the configured system meets all requirements:
- User acceptance criteria
- Regulatory compliance requirements
- Performance under various conditions
- Exception handling procedures
Test scripts should cover both standard operations and edge cases to ensure system reliability.
Validation Protocol Execution
The validation process follows a structured methodology:
- Installation Qualification (IQ) – verifies proper system installation
- Operational Qualification (OQ) – confirms system functions according to specifications
- Performance Qualification (PQ) – demonstrates consistent performance under real-world conditions
Each validation activity must be thoroughly documented to satisfy pharma regulatory compliance requirements.
Data Migration
For manufacturers transitioning from legacy systems, data migration requires careful planning:
- Identification of critical historical data
- Data cleaning and standardization
- Migration validation protocols
- Verification of data integrity after transfer
Proper data migration ensures continuity of operations and maintains the integrity of manufacturing records.
Phase 4: Training and Deployment
The human element plays a decisive role in MES implementation success, making training and deployment among the most consequential MES implementation steps.
Even the most perfectly configured system will fail to deliver value if users don’t understand how to operate it correctly or resist adoption.
This phase bridges the gap between technical configuration and practical operation in your manufacturing environment.
Training for an MES deployment in FDA-regulated industries must go beyond basic software operation. Users need to understand the regulatory context of their actions within the system, the importance of data integrity, and how their interactions affect downstream compliance.
The MES implementation steps for training should include both system mechanics and the underlying quality principles that govern pharmaceutical and medical device manufacturing. Proper documentation of training completion becomes part of the overall system validation package.
User Training
Comprehensive training programs should address different user groups:
- Operators – daily system interaction
- Supervisors – exception handling and approvals
- Quality personnel – compliance monitoring
- IT staff – system maintenance and troubleshooting
Training effectiveness directly impacts user adoption and manufacturing process improvement outcomes.
Phased Rollout
A phased deployment approach minimizes operational disruption:
- Pilot implementation in a single area
- Evaluation and adjustment based on pilot results
- Sequential deployment across remaining production areas
- Full-scale implementation and optimization
This measured approach allows for course corrections while maintaining manufacturing continuity.
Go-Live Support
The transition to live operation requires enhanced support:
- On-site technical specialists
- Extended helpdesk hours
- Daily status meetings
- Rapid issue resolution protocols
Proper support during this critical period builds user confidence and accelerates adoption.
Phase 5: Continuous Improvement
The final MES implementation steps focus on optimization and evolution, transforming the system from a static installation to a dynamic platform that evolves with your manufacturing operations.
While many organizations consider implementation complete after go-live, this approach fails to capture the full potential value of the MES investment. The continuous improvement phase establishes mechanisms for ongoing refinement based on operational experience and changing business requirements.
This phase of MES implementation steps requires formalized processes for collecting user feedback, analyzing system performance data, and prioritizing enhancement opportunities. For FDA-regulated manufacturers, these improvements must balance the need for innovation with regulatory change control requirements.
Each system modification must undergo appropriate validation to ensure continued compliance. Organizations that excel at this phase typically establish a dedicated MES center of excellence with representation from operations, quality, IT, and regulatory affairs.
Performance Monitoring
Systematic monitoring ensures the system delivers expected benefits:
- Key performance indicators (KPIs) tracking
- User feedback collection
- System performance metrics
- Compliance effectiveness assessment
Regular monitoring identifies opportunities for further manufacturing efficiency improvements.
Ongoing Optimization
The MES implementation journey continues with incremental enhancements:
- Regular system reviews and updates
- Process refinements based on operational data
- Additional module implementations
- Integration with new technologies
Organizations like GMP Pros provide specialized data science and analytics services to maximize long-term MES value.

Key Challenges in MES Implementation Steps
Even with careful planning, MES implementations face common obstacles:
Change Management
User resistance often presents the greatest challenge. Effective change management strategies include:
- Early stakeholder engagement
- Clear communication of benefits
- Recognition of implementation team contributions
- Visible executive support
These approaches minimize resistance and accelerate technology adoption.
System Integration
Integration complexity frequently exceeds initial estimates. Success factors include:
- Detailed interface specifications
- Comprehensive testing protocols
- Phased integration approach
- Specialized integration expertise
For complex environments, partners with MES implementation in pharma experience provide valuable guidance.
Validation Documentation
The documentation burden for validated systems can be substantial. Efficiency strategies include:
- Template-based documentation
- Risk-based validation approaches
- Electronic documentation systems
- Clear traceability matrices
These approaches maintain compliance while reducing implementation timelines.
Specialized Considerations for SAP MES Implementation
Organizations pursuing SAP MES implementation face unique considerations:
- Integration with existing SAP ERP environments
- Configuration of industry-specific modules
- Alignment with SAP validation frameworks
- Migration from legacy SAP manufacturing modules
These projects benefit from specialized experience with both SAP environments and FDA-regulated manufacturing.
MES Implementation Project Plan Essentials
A comprehensive MES implementation project plan incorporates several critical elements:
- Realistic timelines with appropriate contingencies
- Resource allocation across multiple departments
- Risk assessment and mitigation strategies
- Clear governance structure and decision processes
- Budget monitoring and control mechanisms
The project plan serves as the roadmap for all MES implementation steps, guiding the organization through this complex transformation.
Measuring Implementation Success
Quantifiable metrics demonstrate MES implementation value:
| Success Metric | Measurement Approach |
| Compliance improvement | Reduction in deviations and audit findings |
| Efficiency gains | Decrease in batch release time |
| Data integrity | Elimination of manual data entry errors |
| Process consistency | Reduction in process variability |
| Return on investment | Total benefits compared to implementation costs |
These metrics provide tangible evidence of implementation success and guide continuous improvement efforts.
Final Considerations and Next Steps
The MES implementation steps outlined in this guide provide a framework for success in FDA-regulated environments. Organizations embarking on this journey should:
- Conduct a thorough readiness assessment
- Develop a detailed implementation roadmap
- Secure appropriate resources and expertise
- Establish clear governance and success metrics
With proper planning and execution, MES implementation delivers transformative benefits for regulated manufacturers.
Ready to Transform Your Manufacturing Operations?
If your organization is considering implementing or optimizing a Manufacturing Execution System, GMP Pros offers specialized expertise in MES implementation for FDA-regulated manufacturers. Our teams embed directly with your operations to ensure successful deployment while maintaining regulatory compliance.
Our Electronic Batch Record implementation services help pharmaceutical and biologics manufacturers transform paper-based processes into efficient digital workflows. Contact us today to discuss how we can support your MES implementation journey with our proven expertise in regulated manufacturing environments.
Key Takeaways:
- Successful MES implementation requires thorough assessment, planning, and cross-functional expertise
- Validation represents a critical success factor for FDA-regulated manufacturers
- A phased deployment approach minimizes operational disruption
- Continuous improvement strategies maximize long-term MES value
- Specialized partners provide valuable guidance for complex implementations

