Our Services
Electronic Batch Records Implementation
Key Goals of Electronic Batch Records (EBR) Implementation:
1️⃣ Ensure Compliance & Data Integrity – Adhere to FDA 21 CFR Part 11, EU Annex 11, and GMP standards while maintaining accurate, audit-ready electronic records.
2️⃣ Increase Efficiency & Reduce Human Errors – Automate data entry, calculations, and approvals to minimize manual errors and streamline batch processing.
3️⃣ Enhance Traceability & Real-Time Monitoring – Enable end-to-end batch visibility, digital audit trails, and real-time reporting for faster decision-making.
4️⃣ Accelerate Batch Release & Reduce Cycle Times – Reduce review and approval delays by digitizing workflows, leading to faster product release.
5️⃣ Improve Manufacturing Scalability & Integration – Seamlessly connect with MES, ERP, and LIMS systems to enhance process control and support business growth.
Outcomes Come First
Client Focused

Delivering Results
Overseeing all aspects of execution, including project management, business change, design, process engineering, project engineering, and validation— with fully integrated teams at two Top 10 Global Pharma Companies. Reduced batch record quality release cycle times by 60% for pharmaceutical clients implementing eBR solutions.

Delivering Results
Delivering Results at Top 3 Global Pharma Company:
Vial Filling Line:
• Line A – 24% OEE Improvement
• Line B – 5.7% OEE Improvement
• Line C – 38% OEE Improvement
Production Engineering
Key Goals of Production Engineering/Centerlining:
1️⃣ Optimize Equipment & Process Performance – Establish and maintain optimal machine settings and process parameters to ensure consistent, high-quality production.
2️⃣ Reduce Variability & Improve Product Consistency – Minimize deviations by standardizing best operating conditions, leading to fewer defects and less rework.
3️⃣ Increase Efficiency & Reduce Waste – Improve machine uptime, reduce material loss, and enhance energy efficiency through data-driven process control.
4️⃣ Enhance Data-Driven Decision-Making – Use real-time monitoring and analytics to track key performance indicators (KPIs) and make proactive adjustments.
5️⃣ Ensure Compliance & Continuous Improvement – Maintain GMP, FDA, and industry regulations while continuously refining processes for better efficiency and cost savings.
Capital Project Engineering
Key Goals of Capital Project Engineering:
1️⃣ Deliver Projects On Time & Within Budget – Ensure efficient execution, cost control, and timely completion.
2️⃣ Ensure Regulatory & Safety Compliance – Adhere to industry standards (FDA, OSHA, EPA, GMP) and implement strong safety protocols.
3️⃣ Optimize Facility & Equipment Performance – Design scalable, efficient systems that enhance production and reliability.
4️⃣ Improve Operational Efficiency & Productivity – Reduce downtime, streamline workflows, and integrate advanced technology.
5️⃣ Manage Risk & Drive Business Growth – Identify and mitigate project risks while supporting long-term scalability and sustainability.

Delivering Results
• Delivered a seamless product transfer for a leading product, encompassing project engineering, validation/qualification, and start-up execution.
• Delivered comprehensive site integration engineering, ensuring smooth facility transitions and operational alignment.
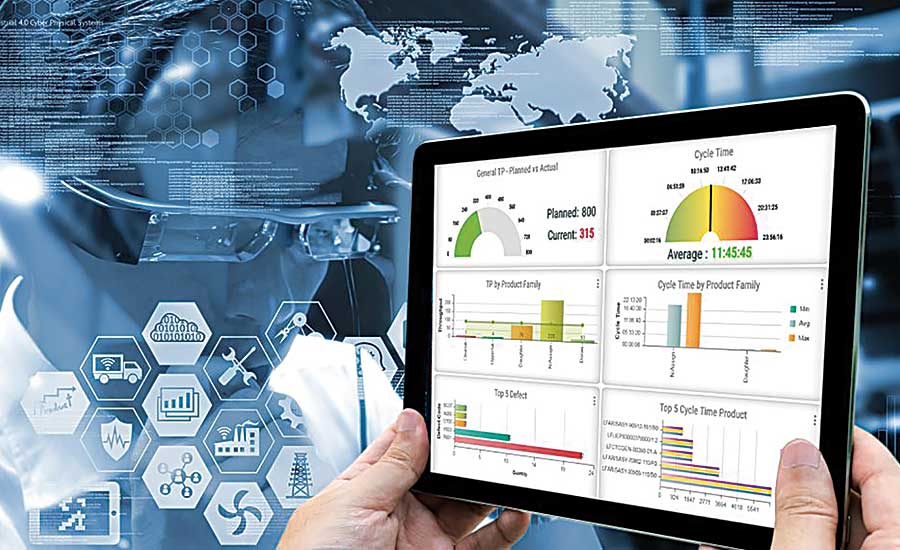
Delivering Results
Developed and standardized Data Science Dashboards and key performance metrics utilized across the North American network for a Top 10 Global Pharma Company.
Data Science & Analytics
Key Goals of Data Science & Analytics:
1️⃣ Enhance Product Quality & Compliance – Use real-time monitoring and predictive analytics to ensure products meet FDA, GMP, and regulatory standards, reducing deviations and batch failures.
2️⃣ Optimize Manufacturing Efficiency & Throughput – Analyze process data to reduce downtime, increase yield, and improve resource utilization, leading to cost savings and operational excellence.
3️⃣ Enable Predictive Maintenance & Reduce Equipment Failures – Implement machine learning models to predict equipment breakdowns, allowing for proactive maintenance and minimizing unplanned downtime.
4️⃣ Improve Supply Chain & Inventory Management – Leverage data analytics for demand forecasting, supplier optimization, and raw material tracking, ensuring fewer stockouts and production delays.
5️⃣ Drive Continuous Improvement & Process Optimization – Use process analytics to identify bottlenecks, enhance process control, and support continuous improvement initiatives in manufacturing.

Achieve Success
At GMP Pros, we offer a range of engineering and technical services, all designed to solve the things that matter. Whether you’re looking for gains in efficiency through process improvement, embedded technical professionals to work directly with your teams, or end to end project solutions, we have you covered. We provide the right people. With the right solutions.
